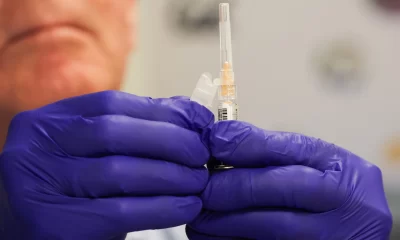
Lawyers of 35 victims of the AstraZeneca Covid vaccine have demanded a whopping £80 million pounds in compensation for the injuries sustained after taking the vaccine.
The cases highlight what is claimed to be a very rare side effect that has been linked to the deaths of at least 81 people and caused serious harm to hundreds more.
The claims allege the vaccine, developed by AstraZeneca in partnership with the University of Oxford, is “defective”.
The vaccine has been linked to a newly identified condition that causes blood clots called Vaccine-Induced Immune Thrombocytopenia and Thrombosis (VITT). In about one-in-five cases, patients who contracted VITT died.
It was gathered that up to 40 more claims are expected to be lodged. The total compensation bill, should AstraZeneca lose, amounts to about £80m, making it one of the most expensive vaccine litigation cases ever.
READ ALSO: Adjuvants in COVID vaccines may lead to serious respiratory disease –experts
The Government has underwritten any legal action brought against AstraZeneca as part of its deal in rolling out the Covid-19 vaccine programme, which received regulatory approval at the end of December 2021. Manufacturers of the other Covid vaccines have also received Government indemnification.
Under the Government’s Vaccine Damage Payment scheme, people who suffer adverse reactions that lead to death or a 60 per cent disability are entitled to a one-off payment of £120,000 tax free. But lawyers argue that sum is wholly inadequate in many of the cases where people have died or been forced to give up lucrative careers.
The scheme has paid out in about 150 cases, of which all but a handful occurred after receiving the AstraZeneca jab. Sir Jeremy maintained that in cases where the Government had already paid damages, causation had effectively been proven.
One of the new cases has been lodged by the widower of Nicola Weideling, an employee of Oxford University, who died aged 45 in May 2021 after having the Oxford-AstraZeneca vaccine. A coroner has already ruled that the cause of death was VITT.
READ ALSO: Adjuvants in COVID vaccines may lead to serious respiratory disease –experts
Her husband Kurt has urged AsatraZeneca to settle the claim. His wife died after developing blood clots and suffering a “significant brain bleed”.
AstraZeneca is fighting the claim. In its defence, it has denied its vaccine was “defective” and has insisted the claims against the company are “confused” and “wrong in law”. The pharmaceutical giant is being sued under the Consumer Protection Act amid claims the vaccine was not as safe as recipients were led to believe.
AstraZeneca points out the vaccine is estimated to have saved six million lives in its first year of rollout. The vaccine is no longer used in the UK as part of the booster programme.
Mr Hollobone started to feel unwell at the end of February 2021. The symptoms occurred after an AstraZeneca Covid vaccine, which he had taken to protect more vulnerable family members and because he believed it offered a “way out” of lockdown.
At first, his symptoms were similar to a bad cold, feeling cold and shivering. Later, he developed extreme headaches, a rash on his leg and a limp.
He was admitted to hospital and – wrongly – diagnosed with appendicitis. Mr Hollobone, who worked as an engineer, was then transferred to another local hospital for further treatment, which was complicated because he also had low platelets, meaning he was at risk of bleeding.
His wife kissed him goodbye and said she would be right behind him. Unfortunately, because of lockdown rules at the time, it was the last time she saw him.
READ ALSO: COVID vaccine may cause long-term heart damage, study reveals
A year after Mr Hollobone died, a coroner concluded that his death “was caused by the vaccination”. His death certificate listed blocked blood flow to his bowel, blood clot and vaccine-induced thrombocytopenia and thrombosis as the causes of death. The latter is now known as VITT.
A spokesperson for AstraZeneca has previously said patient safety was its “ “highest priority””, that its vaccine, called Vaxzevria, had “ “continuously been shown to have an acceptable safety profile”” and that regulators around the world “ “consistently state that the benefits of vaccination outweigh the risks of extremely rare potential side effects””.
In response to previous cases which have been lodged, the company has denied any liability and has insisted that the vaccine is not “ “defective”
 Health6 days ago
Health6 days ago
 Entertainment1 week ago
Entertainment1 week ago
 Crime6 days ago
Crime6 days ago
 Education1 week ago
Education1 week ago
 Health1 week ago
Health1 week ago
 Comments and Issues7 days ago
Comments and Issues7 days ago
 Football1 week ago
Football1 week ago
 Latest7 days ago
Latest7 days ago