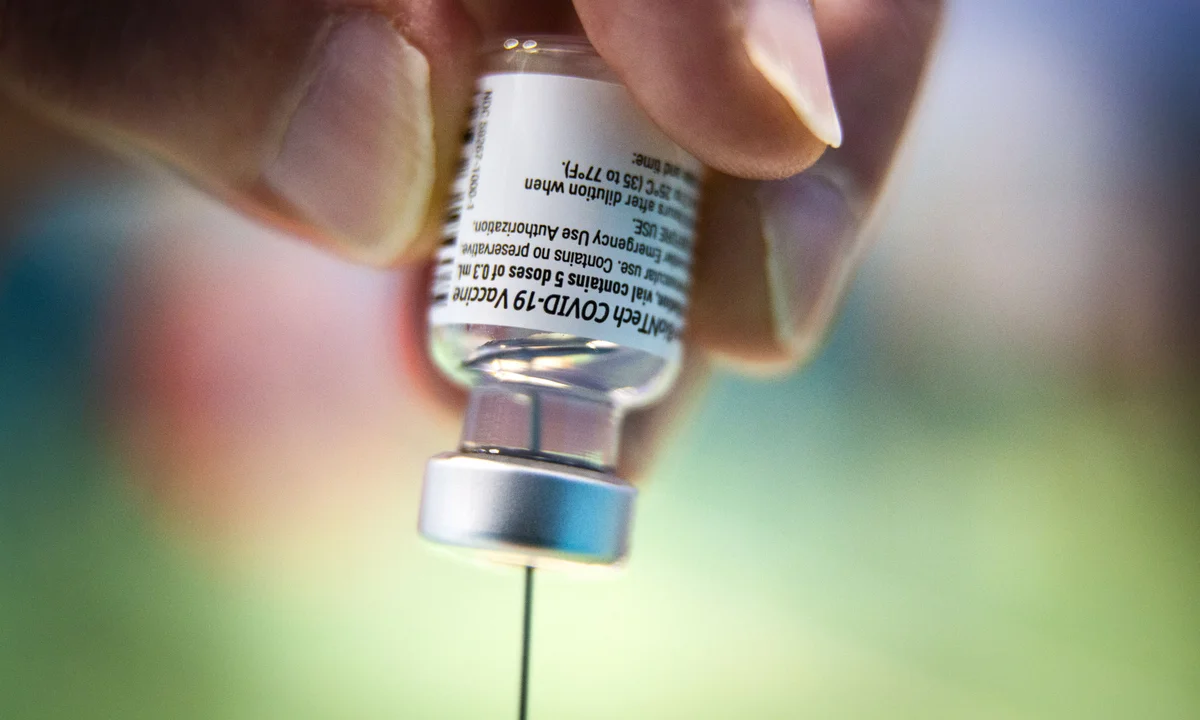
Newly released data obtained through a Freedom of Information (FOI) request have shown that tens of thousands of cardiac-related adverse events were reported to the U.K.’s Medicines and Healthcare products Regulatory Agency (MHRA) following administration of the AstraZeneca COVID-19 vaccine during the early phase of the pandemic rollout.
According to the data, in 2021 alone, the MHRA received 48,472 reports of adverse events associated with heart conditions linked to the AstraZeneca vaccine.
Of this figure, 23,914 cardiovascular-related reports were submitted by late March 2021, indicating that nearly half of the cases were reported within the first three months of the vaccine’s public rollout.
During the same period, 6,175 reports of blood-clotting (thromboembolic) events were also recorded.
The disclosures were first reported by GB News and stem from an FOI request submitted in October 2025 by Oxford researchers Dr. Tom Jefferson and Dr. Carl Heneghan.
The request sought detailed information on cardiovascular and thromboembolic adverse events associated with the AstraZeneca vaccine—also known as Vaxzevria, a non-mRNA vaccine developed with Oxford University—between February 2021 and January 2024.
The MHRA released the data approximately one month later. Jefferson and Heneghan subsequently analysed the figures and published their findings in a series of Substack posts, describing the disclosures as unprecedented.
“To the best of our knowledge, this is the first time anyone outside official institutions has seen the reports submitted to the MHRA regarding serious potential harms during the early rollout period,” the researchers wrote.
The data appear to support the conclusions of a preprint study by researchers affiliated with Children’s Health Defense (CHD) and the Brownstone Institute, published on Preprints.org.
That study reanalysed existing vaccine safety data and argued that earlier studies had failed to compare risks across different COVID-19 vaccines, potentially obscuring safety signals.
Karl Jablonowski, Senior Research Scientist at CHD, criticised the MHRA’s interpretation of its own data, alleging that regulators presented conclusions that conflicted with reported adverse event trends.
READ ALSO: New study links COVID-19 vaccines to multiple cancer types
“Instead of acknowledging the scale of cardiovascular harm suggested by their own data, officials offered reassurance about vaccine safety,” Jablonowski said, adding that the approach undermined public trust.
Following publication of the researchers’ analysis, the MHRA reportedly revised its figures. The agency later stated that the number of heart-condition reports linked to the AstraZeneca vaccine during the same period was 13,010, nearly four times lower than the original figure disclosed.
An MHRA spokesperson told GB News that the agency is reviewing previously released data to identify potential discrepancies.
Commenting on the controversy, TrialSite News noted that while adverse event reporting systems are designed to detect safety signals rather than establish causation, large inconsistencies can weaken confidence in regulatory risk communication.
The FOI request also sought information on the total number of AstraZeneca doses administered in the U.K. Initially, the UK Health Security Agency declined to release the data, citing commercial sensitivity.
However, the figures were later disclosed following an appeal. According to the researchers, the data showed a strong correlation between doses administered and adverse event reports. They also noted that reports continued even after the vaccine was withdrawn, suggesting what they described as a possible “long-term dose effect.”
Daniel O’Connor, Founder and CEO of TrialSite News, said the disclosures highlight broader regulatory issues during the pandemic.
“The issue is not only the adverse events themselves, but why their full scale emerged only through FOI requests years later,” O’Connor said. “Delayed risk disclosure compromises informed consent and erodes trust.”
The AstraZeneca vaccine was withdrawn from the market in 2024, officially for commercial reasons. However, court filings submitted in the U.K. that year acknowledged that the vaccine could, in very rare cases, cause blood clots.
This admission has since become central to a growing class-action lawsuit involving individuals who allege serious, life-altering injuries.
During the same period, several European countries suspended or withdrew the vaccine, and regulators later advised that younger adults be offered alternative vaccines.
The MHRA has maintained that adverse event reports do not establish causation and that regulatory decisions were based on the totality of available evidence at the time. Nonetheless, the latest disclosures have reignited debate over transparency, risk communication, and accountability in pandemic-era public health regulation.
 Health5 days ago
Health5 days ago
 Entertainment7 days ago
Entertainment7 days ago
 Crime6 days ago
Crime6 days ago
 Education1 week ago
Education1 week ago
 Health1 week ago
Health1 week ago
 Comments and Issues7 days ago
Comments and Issues7 days ago
 Football7 days ago
Football7 days ago
 Latest6 days ago
Latest6 days ago