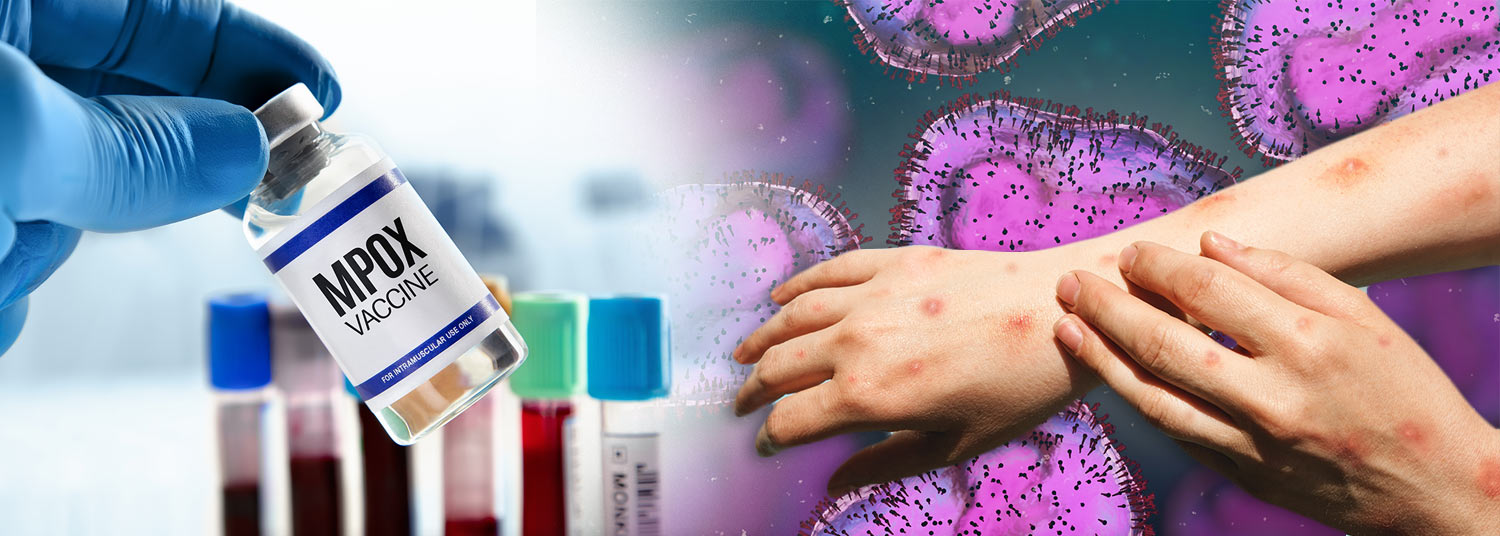
The U.S. Food and Drug Administration (FDA) recently expanded the approval of the ACAM2000 vaccine for use against mpox, sparking concerns due to potential risks.
Initially approved for smallpox in 2007, ACAM2000, made by Emergent BioSolutions, was cleared for high-risk mpox patients on August 29, 2023.
However, its FDA medication guide warns of serious complications, including myocarditis, pericarditis, and fetal death, which may affect vaccinated individuals and their close contacts up to six weeks after receiving the shot.
Critics, like Karl Jablonowski, Ph.D., of Children’s Health Defense, argue that the approval poses significant public health risks, with estimates suggesting that widespread vaccination could result in thousands of cases of myopericarditis, a severe heart condition.
READ ALSO: MPOX: APC UK South-West caucus task Minister of Health, others on curtailment
The vaccine’s use of a live vaccinia virus also raises concerns about its transmission to others, leading to potentially fatal outcomes, especially for vulnerable groups such as pregnant women and infants.
Additionally, the shot contains a “live vaccinia virus” that can be spread to — and possibly cause the death of — people who have close contact with the vaccinated person up to 6 weeks following vaccination, according to the medication guide.
The guide — which the FDA requires to be given to patients before they get the ACAM2000 vaccine — says, “ACAM2000 can cause serious complications in vaccinated individuals and in their close contacts to whom the vaccine virus has spread.”
Jablonowski said, “The spread of vaccinia virus is an infliction and an assault, as an unsuspecting person cannot possibly consent.”
“For good reasons you would not consent to this vaccine,” Jablonowski said, “even if you believed your benefits outweigh your risks, as you become a potential vector of a disease that is damaging to the heart and fatal to both fetus and infant.”
Although the U.S. maintains over 100 million doses of ACAM2000, health authorities emphasize that it has not been broadly recommended for civilian use during the ongoing mpox outbreak, with the alternative vaccine JYNNEOS preferred due to fewer side effects.
 Health1 week ago
Health1 week ago
 Latest1 week ago
Latest1 week ago
 Health7 days ago
Health7 days ago
 Football7 days ago
Football7 days ago
 News1 week ago
News1 week ago
 Latest1 week ago
Latest1 week ago
 News1 week ago
News1 week ago
 Latest1 week ago
Latest1 week ago