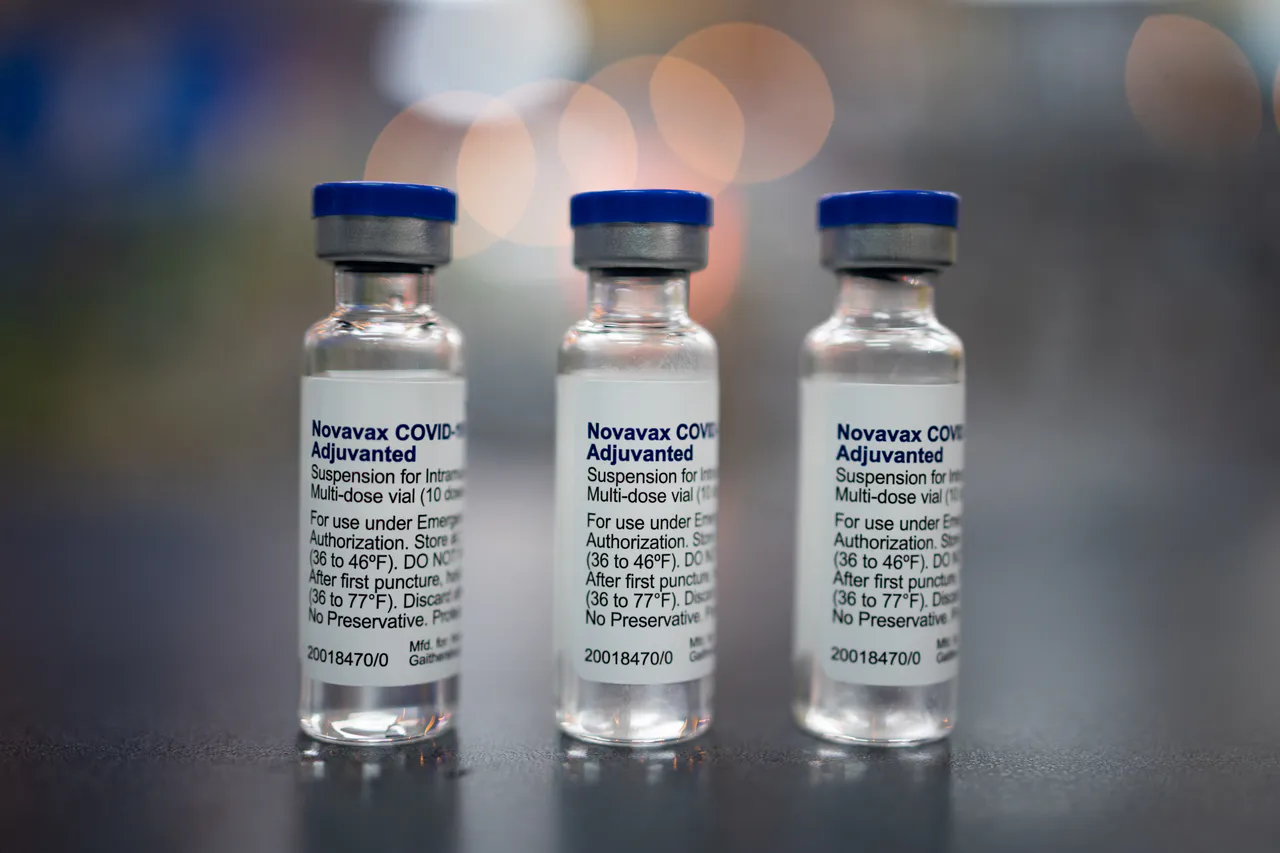
The U.S. Food and Drug Administration (FDA) have halted trials for Novavax’s experimental COVID-19-flu combination vaccine and its standalone flu shot after a trial participant reported nerve damage.
The participant, who received the COVID-flu vaccine during a Phase 2 trial in January 2023, later developed motor neuropathy — a condition that affects the nerves controlling muscles. Novavax said it was only informed of the case last month.
In response, the FDA placed a clinical hold on Novavax’s planned Phase 3 COVID-flu vaccine trial, which was scheduled to begin this month, as well as on its flu vaccine trials.
The company completed its Phase 2 trials last year and maintains that prior trials have not shown any signs of motor neuropathy as a safety concern.
Novavax Chief Medical Officer Robert Walker stated that the company does not believe the reported nerve damage is linked to its vaccine.
“We are working closely with the FDA to provide the necessary information that will allow them to better understand this observation and resolve the clinical hold,” Walker said in a press release.
Despite this, Novavax’s stock dropped sharply, with a 20% decline in early trading following the FDA’s announcement. The company’s future growth is heavily tied to the success of its COVID-flu combination and flu vaccines, making the hold a significant blow to its financial stability.
Novavax has already undergone two rounds of layoffs this year, cutting its workforce by 30%.
The FDA’s suspension of the Novavax trials comes amid growing concerns about how regulatory agencies handle adverse events in clinical trials.
There have been other recent cases where serious side effects were reported in drug trials, yet the FDA allowed those trials to continue, eventually approving the drugs.
For example, Pfizer’s RSV vaccine Abrysvo and GSK’s Arexvy were both linked to Guillain-Barré syndrome in clinical trials, yet the FDA approved the vaccines for adults over 60 in 2023.
The Centers for Disease Control and Prevention (CDC) later restricted the recommended age group for the vaccines to those over 75, citing the increased risk of adverse events, including Guillain-Barré syndrome.
Brian Hooker, Ph.D., chief scientist for Children’s Health Defense, expressed concern over the inconsistency in the FDA’s approach to clinical trials.
“While it’s good that the Novavax trial was paused, the FDA needs to explain why some trials with serious adverse events, including deaths, are allowed to continue while others are halted,” Hooker said.
He criticized the influence of corporate funding on regulatory decisions, saying, “Industry money flowing through programs like Fast Track creates pressure on the FDA to approve products prematurely, putting patients at risk.”
Novavax, whose only commercial product is its COVID-19 vaccine, has struggled to gain significant market share against competitors like Pfizer and Moderna.
The company’s financial position has become increasingly precarious, with its COVID-19 vaccine underperforming in terms of U.S. administration rates and facing numerous reports of adverse effects, including Guillain-Barré syndrome and other serious conditions.
As the FDA investigates further, Novavax’s ability to move forward with its COVID-flu combination vaccine — a critical product for its future — remains uncertain.
 Entertainment5 days ago
Entertainment5 days ago
 Health7 days ago
Health7 days ago
 Health4 days ago
Health4 days ago
 Football1 week ago
Football1 week ago
 Football1 week ago
Football1 week ago
 Crime4 days ago
Crime4 days ago
 Crime1 week ago
Crime1 week ago
 Education6 days ago
Education6 days ago