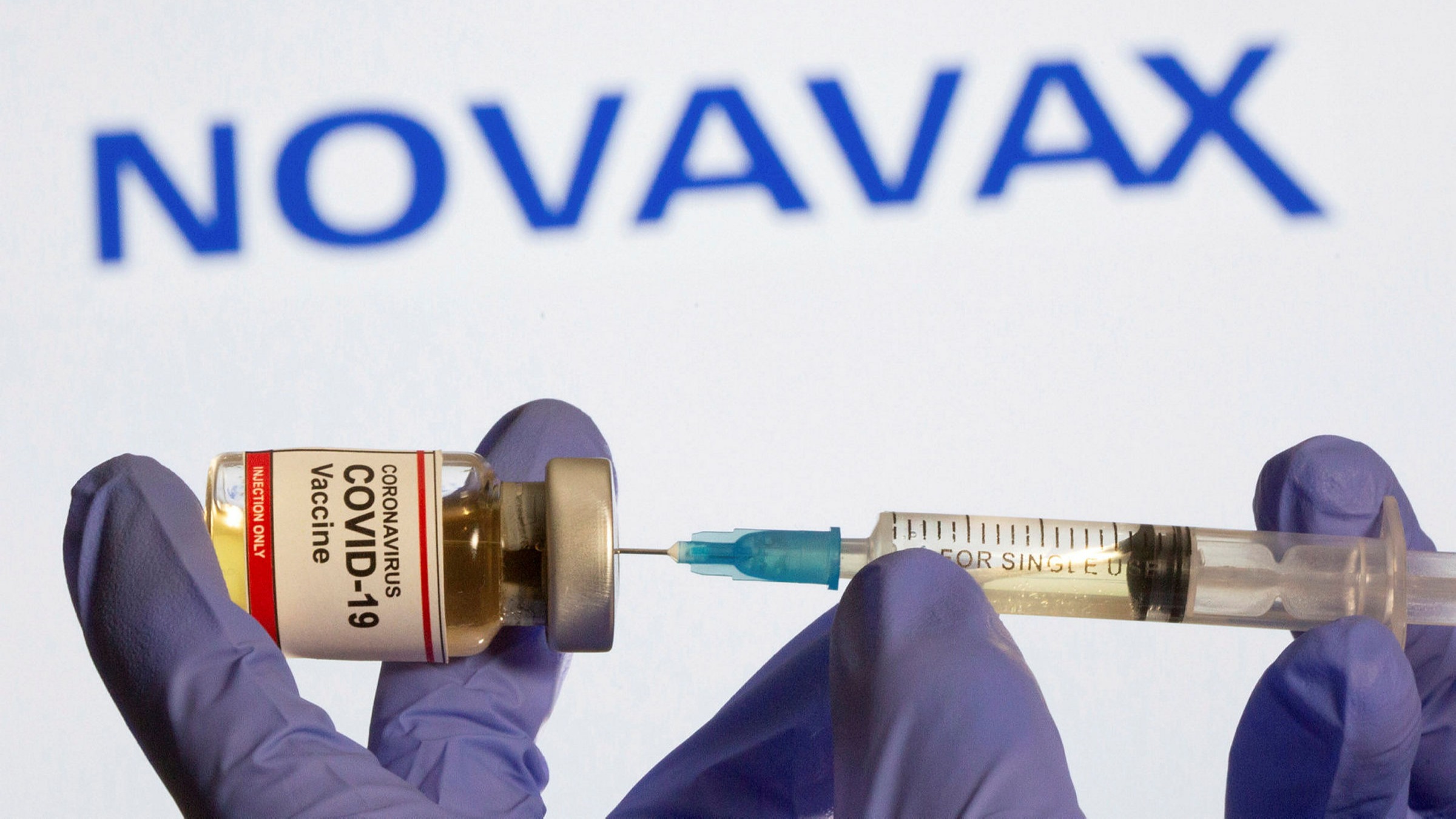
The European Medicines Agency (EMA) has recommended adding a warning for two types of heart inflammation to Novavax’s COVID-19 vaccine, marketed under the brand names Nuvaxovid and Covovax, based on a small number of cases reported in those who received the vaccine.
According to a statement, the EMA’s Pharmacovigilance Risk Assessment Committee — responsible for assessing and monitoring the safety of human medicines — concluded that “myocarditis and pericarditis can occur following vaccination with Nuvaxovid.”
“The Committee is therefore recommending listing myocarditis and pericarditis as new side effects in the product information for Nuvaxovid, together with a warning to raise awareness among healthcare professionals and people receiving this vaccine,” the statement said.
READ ALSO: Family of 27-year-old who died after AstraZeneca shot weighs legal action
The Pharmacovigilance Risk Assessment Committee also requested the “marketing authorization holder of Nuvaxovid provides additional data on risk of side effects occurring.”
According to Reuters, the U.S. Food and Drug Administration (FDA) flagged a risk of heart inflammation from the Novavax vaccine in early June.
However, the agency on July 13 granted Novavax’s request for Emergency Use Authorization of the vaccine for adults 18 and over in the U.S.
Novavax hoped people who opted not to take Pfizer and Moderna mRNA vaccines — both of which are associated with a risk of heart inflammation — would favor its shot because it “relies on technology used for decades,” Reuters reported.
The FDA’s “Fact Sheet for Healthcare Providers Administering Vaccine” now includes a warning that “clinical trial data provide evidence for increased risks of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of tissue surrounding the heart) following administration of Novavax COVID-19 Vaccine, Adjuvanted.”
READ ALSO: Medical experts kick as FDA panel recommends Moderna COVID vaccine for kids
The FDA’s “Fact Sheet for Recipients and Caregivers” states that for most people who have had myocarditis or pericarditis after receiving the vaccine, symptoms began within 10 days following vaccination and that vaccine recipients should seek medical attention right away if they experience chest pain, shortness of breath, feelings of having a fast-beating, fluttering or pounding heart.
Myocarditis is inflammation of the heart muscle that can lead to cardiac arrhythmia and death. According to the National Organization for Rare Disorders, myocarditis can result from infections, but “more commonly the myocarditis is a result of the body’s immune reaction to the initial heart damage.”
Unlike the Pfizer and Moderna vaccines that use mRNA technology, and the Johnson & Johnson (J&J) vaccine that uses adenovirus vector technology, the Novavax vaccine uses a more traditional vaccine technology.
READ ALSO: Deaths, heart attack linked to COVID Vaccines on increase
Despite a unanimous vote by the FDA’s vaccine advisory panel, members raised several concerns about the Novavax vaccine, including heart-related adverse events observed during the clinical trials.
In a safety database encompassing data from 40,000 Novavax vaccine recipients, four young men, ages 16 through 28, reported myocarditis or pericarditis within 20 days of injection, though one of the four had a viral illness that may have caused the symptoms.
One trial participant reported myocarditis after being injected with a placebo.
According to the FDA, there were 26,000 people in the Novavax clinical trial. Yet, only 21,000 of the 26,000 people who received the vaccine during the trial were “followed for at least 2 months.”
 Entertainment5 days ago
Entertainment5 days ago
 Health1 week ago
Health1 week ago
 Health4 days ago
Health4 days ago
 Football1 week ago
Football1 week ago
 Football1 week ago
Football1 week ago
 Crime4 days ago
Crime4 days ago
 Education6 days ago
Education6 days ago
 Crime1 week ago
Crime1 week ago