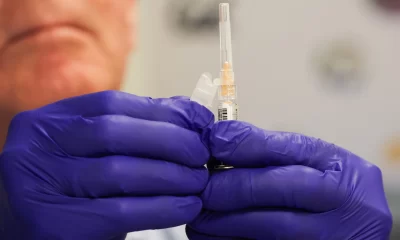
The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a stern warning to counterfeiters and purveyors of substandard food and drug products, declaring that Nigeria will not serve as a dumping ground for unsafe goods.
The agency revealed strengthened international collaborations to ensure that only quality products are shipped into the country, particularly from major exporting nations like China and India.
Speaking at the 8th Annual Conference of the Association of Nigerian Health Journalists (ANHEJ) in Abuja, NAFDAC emphasized that pre-shipment testing protocols are now being enforced as a critical safeguard.
Representing the agency’s Director General, the Director of Marketing Surveillance highlighted that NAFDAC has appointed independent analysts in key export countries to rigorously test products before they are dispatched to Nigeria.
This measure, the agency noted, aims to curtail the entry of counterfeit and substandard items while protecting consumers and local businesses.
“Pre-shipment testing ensures that only quality products are sent to Nigeria. We have appointed independent analysts in these countries to verify product safety before they leave, reducing the risk of waste and counterfeit items entering our markets,” the Director of Marketing Surveillance stated.
The agency also announced that 144 batches of substandard pharmaceutical products were recently intercepted and prevented from entering the Nigerian market.
Such successes are part of NAFDAC’s comprehensive strategy, which combines routine post-marketing surveillance, consumer complaints, and technological tools to target high-risk products.
READ ALSO: NAFDAC, PCN renew commitment to eradicate open drug markets
“We are not a dumping ground for substandard products,” the official reiterated. “We have adopted innovative tools in collaboration with the United States Pharmacopeia (USP) and the World Health Organization (WHO) to identify high-risk areas for product sampling.”
NAFDAC underscored the importance of aggressive post-marketing inspections, given that counterfeiters often seek to bypass regulatory oversight.
These inspections, combined with strengthened pre-shipment testing, have significantly reduced the circulation of harmful food and drug products.
In addition to its regulatory efforts, NAFDAC encouraged Nigerians to remain vigilant by reporting suspicious or counterfeit products.
It also urged the promotion of local pharmaceutical manufacturing to reduce Nigeria’s reliance on imports and mitigate the risks associated with foreign products.
The agency’s initiatives reflect a broader push to ensure consumer safety and support Nigeria’s domestic industries by stemming the tide of substandard goods.
 Health6 days ago
Health6 days ago
 Entertainment1 week ago
Entertainment1 week ago
 Crime7 days ago
Crime7 days ago
 Comments and Issues1 week ago
Comments and Issues1 week ago
 Football1 week ago
Football1 week ago
 Latest7 days ago
Latest7 days ago
 Comments and Issues1 week ago
Comments and Issues1 week ago
 Comments and Issues1 week ago
Comments and Issues1 week ago

