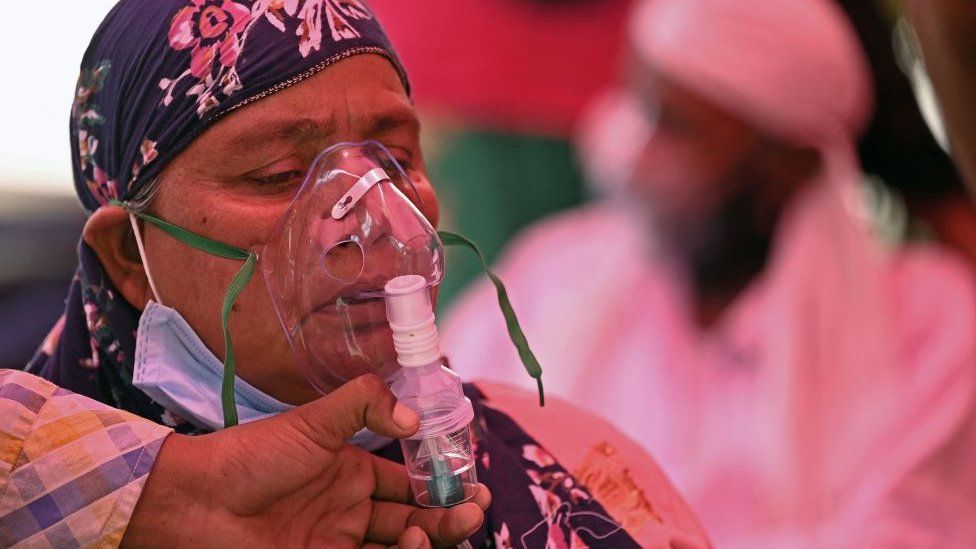
VAERS data released Friday by the Centers for Disease Control and Prevention show 1,261,149 reports of adverse events from all age groups following COVID-19 vaccines, including 27,968 deaths and 228,477 serious injuries between Dec. 14, 2020, and May 6, 2022.
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
The data included a total of 27,968 reports of deaths — an increase of 210 over the previous week — and 228,477 serious injuries, including deaths, during the same time period — up 1,774 compared with the previous week. There were 5,794 additional total adverse events reported to VAERS over the previous week.
READ ALSO: Pilots injured by COVID vaccines speak out
Excluding “foreign reports” to VAERS, 815,384 adverse events, including 12,899 deaths and 81,830 serious injuries, were reported in the U.S. between Dec. 14, 2020, and May 6, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 12,899 U.S. deaths reported as of May 6, 16% occurred within 24 hours of vaccination, 20% occurred within 48 hours of vaccination and 59% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 578 million COVID-19 vaccine doses had been administered as of May 6, including 341 million doses of Pfizer, 218 million doses of Moderna and 19 million doses of Johnson & Johnson (J&J).
Second and third doses of Pfizer’s COVID-19 vaccine provide protection against the Omicron variant for only a few weeks, according to peer-reviewed research published today in JAMA Network Open.
READ ALSO: How COVID vaccine destroyed Surgeon’s 20-year career
“Our study found a rapid decline in Omicron-specific serum neutralizing antibody titers only a few weeks after the second and third doses of [the Pfizer-BioNTech] BNT162b2,” the authors of the research letter wrote.
The authors said their findings “could support rolling out additional booster shots to vulnerable people as the variant drives an uptick in new cases across the country,” Forbes reported.
Danish researchers studied adults who received two or three doses of BNT162b2 between January 2021 and October 2021, or were previously infected prior to February 2021 and then vaccinated.
They found that after an initial increase in Omicron-specific antibodies after the second Pfizer shot, levels dropped rapidly, from 76.2% at week 4, to 53.3% at weeks 8 to 10, and 18.9% at weeks 12 to 14.
After the third shot, neutralizing antibodies against Omicron fell 5.4-fold between week 3 and week 8.
The FDA’s top vaccine official told a congressional committee on May 6 that COVID-19 vaccines for kids under 6 will not have to meet the agency’s 50% efficacy threshold for blocking symptomatic infections required to obtain Emergency Use Authorization.
“If these vaccines seem to be mirroring efficacy in adults and just seem to be less effective against Omicron like they are for adults, we will probably still authorize,” Dr. Peter Marks, director of the Center for Biologics Evaluation and Research at the FDA told the House Select Subcommittee on the Coronavirus Crisis.
The FDA is reviewing data from Moderna’s two-shot vaccine for infants and toddlers 6 months to 2 years old, and for children 2 to 6 years old. The company asked the FDA on April 28 to approve its COVID-19 mRNA-1273 vaccine for children, citing different efficacy numbers than it disclosed in March.
The FDA is still awaiting data on Pfizer and BioNTech’s three-dose regimen for children under age 5 after two doses of its pediatric vaccine failed to trigger an immune response in 2-, 3- and 4-year-olds comparable to the response generated in teens and adults.

 Entertainment7 days ago
Entertainment7 days ago
 Entertainment4 days ago
Entertainment4 days ago
 Comments and Issues6 days ago
Comments and Issues6 days ago
 Business7 days ago
Business7 days ago
 Comments and Issues6 days ago
Comments and Issues6 days ago
 Health1 week ago
Health1 week ago
 Health5 days ago
Health5 days ago
 Editorial Opinion1 week ago
Editorial Opinion1 week ago