Some 29 million doses of AstraZeneca COVID-19 vaccines were found during an inspection at a plant in Italy over the weekend, a French official said on Wednesday.
The officials said that checks were still needed to assess whether they were export-bound.
The news of the AstraZeneca doses hidden at a Catalent factory in Anagni, near Rome, was first reported earlier on Wednesday by Italian newspaper, La Stampa, which said the doses were found in an inspection by Italian authorities after a request from the European Commission.
The discovery may well deepen an EU standoff with AstraZeneca which has slashed its supply targets to the bloc and with Britain which wants access to some AstraZeneca doses produced in the EU.
“An inspection took place this weekend at the Anagni plant and uncovered a stock of 29 million doses.
“The destination for these doses still needs to be clarified,’’ the official at the French presidency said, noting that if doses were for export, they could be blocked.
An Italian official confirmed the inspection and said the doses were to be sent to Belgium.
It is unclear whether from Belgium they could be exported or distributed across the EU.
The European Commission declined to comment on the doses at the Anagni factory, a spokesman for AstraZeneca did not reply to a request for comment, Catalent was not immediately available for comment.
An EU official said some of the doses at the Catalent plant might come from a vaccine factory in the Netherlands run by AstraZeneca’s subcontractor Halix.
The Anagni plant is in charge of bottling AstraZeneca vaccines produced at the Halix factory and also at a plant in Belgium run by subcontractor Thermo Fisher Scientific.
Both vaccine-making factories are listed in the contract AstraZeneca signed with the EU in August as suppliers to the EU.
AdvertisementHowever, the Halix factory has not yet been approved in the EU, as AstraZeneca did not submit sufficient data to the EU drugs regulator. Vaccines produced there cannot be used in the EU until that approval is received.
It is unclear why AstraZeneca did not seek earlier approval for the factory.
The Anglo-Swedish drug maker declined to comment on this.
Halix is also listed as a supplier for Britain, which is urging the EU to allow the shipment of doses produced there.
Britain has so far exported no AstraZeneca vaccines to the EU, in spite of the two UK plants being listed in the EU contract as suppliers for the bloc.
Halix said it started producing vaccines for the EU in December and had a capacity to produce about five million doses per month.
The company declined to comment on the precise amount of vaccines it had already produced or on their destination.
AstraZeneca said earlier in March it would aim to deliver only 100 million doses to the EU by the end of June, instead of 300 million envisaged in its supply contract with the 27-nation bloc.
The EU blocked, in early March, a shipment of AstraZeneca vaccines to Australia. saying the move was justified by the company’s non-compliance with its contractual obligations with the EU.


 Latest1 week ago
Latest1 week ago
 Aviation5 days ago
Aviation5 days ago
 News2 days ago
News2 days ago
 Featured5 days ago
Featured5 days ago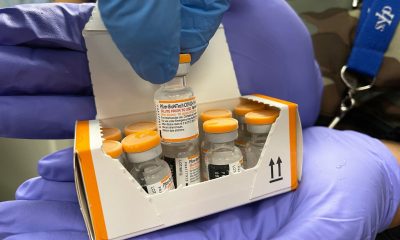
 Covid-196 days ago
Covid-196 days ago
 News1 week ago
News1 week ago
 Featured3 days ago
Featured3 days ago
 Crime3 days ago
Crime3 days ago



