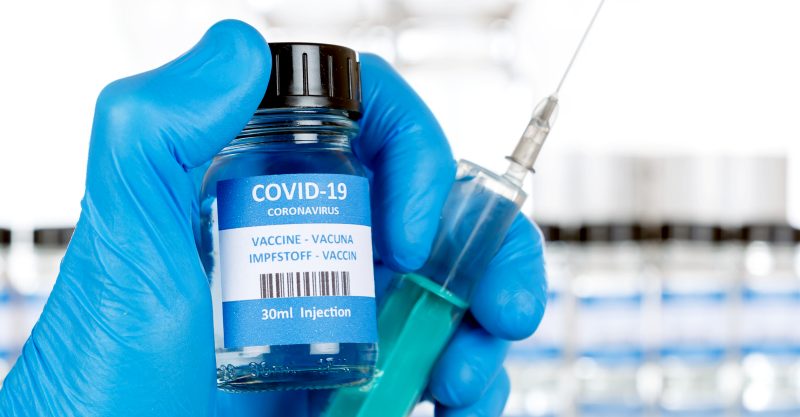
A British study into whether doses of the AstraZeneca/Oxford and Pfizer/BioNTech Coronavirus (COVID-19) vaccines can be safely mixed for two-step vaccines is adding the Moderna and Novavax jabs to its research.
The scientists behind the Com-Cov study, which was launched in February and is being led by the University of Oxford, announced on Wednesday the two jabs would be included in the study.
It is recruiting adults aged 50 and older who have received their first vaccination in the last eight to 12 weeks.
A further 1,050 people are being added to the study.
The idea of the study is to “explore whether the multiple COVID-19 vaccines that are available can be used more flexibly,’’ according to Matthew Snape, who is leading the trial.
Volunteers who had received either the Oxford/AstraZeneca or the Pfizer/BioNTech vaccine will randomly be given either the same vaccine for their second dose or a dose of the jabs produced by Moderna or Novavax.
Currently, the Oxford/AstraZeneca, the Pfizer/BioNTech, and the Moderna vaccines are being administered to people in England and Wales, while Scotland and Northern Ireland are receiving the two former vaccines.
People who are aged 29 and younger, however, have been told to receive the Pfizer/BioNTech or the Moderna vaccines instead of the Oxford/AstraZeneca, after reports of blood clots were received by regulators in Britain.
Results from the original trial, involving AstraZeneca and Pfizer shots, are expected later this month or in May.
The results of the second phase with the Moderna and Novavax doses should come in July.

 Football1 week ago
Football1 week ago
 Health & Fitness2 days ago
Health & Fitness2 days ago
 Comments and Issues1 week ago
Comments and Issues1 week ago
 Featured6 days ago
Featured6 days ago
 Education7 days ago
Education7 days ago
 Business7 days ago
Business7 days ago
 Crime7 days ago
Crime7 days ago
 Business6 days ago
Business6 days ago