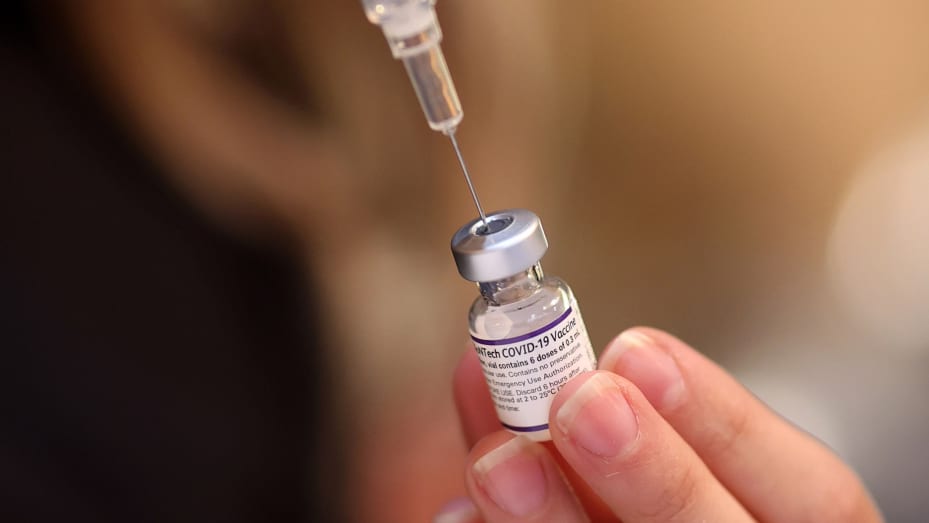
Medical experts have kicked against approval of a new COVID-19 vaccine produced by Pfizer-BioNTech and Moderna for Infants as Young as 6 months, noting that it is unethical to promote these boosters as safe and effective when it is clear they are not.
The U.S. Food and Drug Administration (FDA) on Monday approved updated mRNA COVID-19 vaccines produced by Pfizer-BioNTech and Moderna, paving the way for their approval by the Centers for Disease Control and Prevention (CDC).
Linda Wastila, Ph.D., professor of geriatric pharmacotherapy at the University of Maryland School of Pharmacy and director of research for the Peter Lamy Center for Drug Therapy and Aging, accused the FDA of acting unethically.
“The fact that these vaccines were authorized for children when a public health emergency no longer exists is unconscionable.”
Dr. Pierre Kory, president and chief medical officer of the Front Line COVID-19 Critical Care Alliance (FLCCC), said,”It is unconscionable that the government can recommend this booster for 6-month-olds when the FDA has no data on how children might be affected.”
In a separate statement, the FLCCC said, “By leveraging the unnecessary Emergency Use Authorization … the FDA is pushing forward with an experimental vaccine booster based on limited safety and efficacy data.”
“There is no need to vaccinate healthy children for COVID-19,” Kory said. “To give them an untested booster goes against everything we are trained to do as physicians.”
READ ALSO: Why I regretted getting COVID vaccine–Journalist
Canadian physician Dr. William Makis said, “There is no ‘COVID-19 emergency’ for children, therefore there is no legitimate scientific basis for an ‘emergency authorization’ of a new COVID-19 booster in this age group,” adding, “any doctor still administering COVID-19 mRNA vaccines to children of any age is engaging in medical malpractice.”
According to Wastila, “Both Moderna and Pfizer have failed to deliver on promised post-marketing studies” from prior COVID-19 vaccines. “We have yet to see the results from the bivalent vaccine safety studies in pregnant women; the myocarditis studies in young people also have not been completed nor have most results been shared.”
In separate remarks shared with KFF Health News, Offit, who is 72, said “I don’t plan to get it myself,” referring to the updated COVID-19 vaccine.
Dr. Ashish Jha, former White House COVID-19 adviser and now dean of Brown University’s School of Public Health, told KFF that “a reasonable person” could disagree regarding the need for healthy young people to get the updated vaccine.
Risch called the updated COVID-19 vaccines “a useless idea,” noting that “Almost everybody has had COVID-19 at least once and/or has been vaccinated and has a substantial degree of immunity. That puts them at low risk of serious responses to the viruses circulating now and through the fall and winter.”
A survey of 2,196 people published Sept. 4 in Vaccine found that the main reasons for not receiving the previous bivalent booster included immunity due to prior infections, concerns about side effects and a belief that boosters do not provide protection.

 Health & Fitness5 days ago
Health & Fitness5 days ago
 Aviation1 week ago
Aviation1 week ago
 Aviation6 days ago
Aviation6 days ago
 Aviation6 days ago
Aviation6 days ago
 Aviation6 days ago
Aviation6 days ago
 Aviation5 days ago
Aviation5 days ago
 Featured3 days ago
Featured3 days ago
 Crime3 days ago
Crime3 days ago