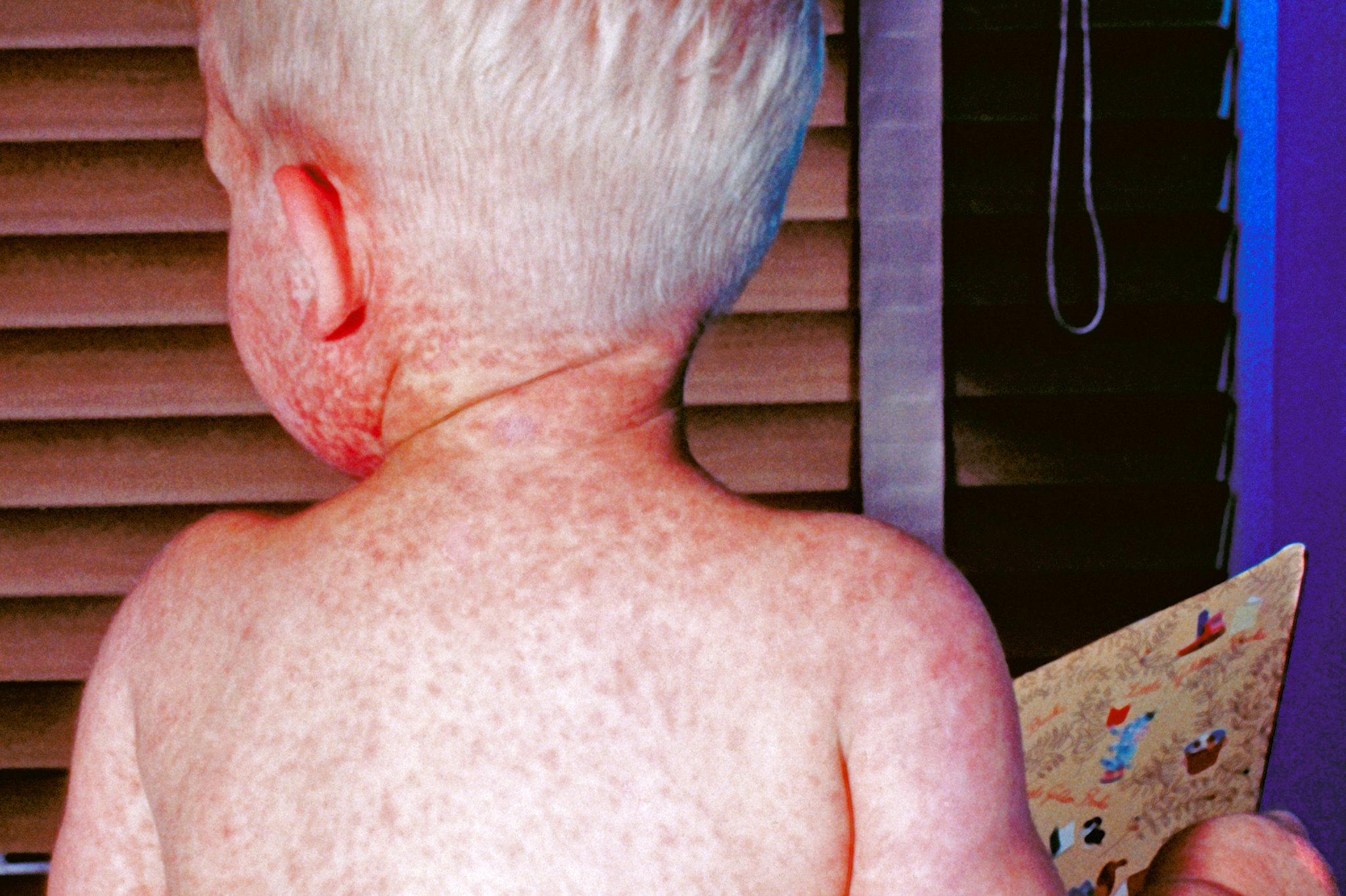
More than 1,000 reports of adverse events have been lodged with U.S. authorities following COVID-19 vaccination in children aged 5 and younger
Most of the adverse event reports have been for outcomes designated non-serious, or events that did not include death, a life-threatening illness, hospitalization or prolongation of hospitalization, permanent disability, congenital anomaly or birth defect.
Of the reported events, 19 were designated serious, with nine for children who received a Moderna vaccine and 10 for children who received a Pfizer vaccine.
“Those details should not have been left out of the information released to ACIP [Advisory Committee on Immunization Practices] and the public,” Barbara Loe Fisher, co-founder and president of the National Vaccine Information Center, told The Epoch Times in an email.
The most commonly reported events after receipt of Pfizer’s vaccine were errors in dosing, such as an incorrect dose being administered. Fever, rash, vomiting, fatigue and diarrhea were also commonly reported. The most commonly reported events after receipt of Moderna’s vaccine were fever, rash, vomiting and hives.
READ ALSO: Study reveals dangers of COVID-19 vaccine on pregnant women, fertility
The number of reports represented 0.06% of the doses administered to children aged 5 or younger, who only became eligible for a vaccine in mid-June.
Shimabukuro, a researcher with the Centers for Disease Control and Prevention (CDC), which runs VAERS with the U.S. Food and Drug Administration, said the data from VAERS and other systems did not reveal any new safety concerns.
Both the Pfizer and Moderna vaccines are under emergency use authorization for young children.
However, research has found that the number of reports submitted to VAERS is an undercount of the actual number of adverse events.
“Even though not every adverse event reported to VAERS is causally related to vaccination, it is also true that one CDC-funded study estimated that less than one percent of vaccine adverse events that occur are reported to VAERS,” Fisher said.
Myocarditis and pericarditis, forms of heart inflammation, were also not detected in the original clinical trials. Studies have since conclusively linked them to the Moderna and Pfizer vaccines.
READ ALSO: COVID vaccine injuries rise as experts raise concerns
The reported rates of myocarditis after dose 2 of a primary series are elevated for males aged 5 to 49 and females aged 12 to 29. The highest reported rate is 78.7 per million second doses administered, for males between 16 and 17 years old.
The reported rates remain elevated for males 12 to 29 after a booster dose, Shimabukuro said. The rates do not remain elevated for females of any age after a booster dose, according to the VAERS data.
The Pfizer vaccine caused 61.7 excess heart inflammation events for males aged 12 to 15 and 189 excess events for males aged 16 and 17. The vaccines caused 30.9 excess events for males aged 18 to 39.
The rates were higher for both males and females aged 16 and 17 after a booster of Pfizer’s shot, though the higher rates were not statistically significant because of the low number of events, Shimabukuro said.
 Entertainment6 days ago
Entertainment6 days ago
 Health1 week ago
Health1 week ago
 Health4 days ago
Health4 days ago
 Football1 week ago
Football1 week ago
 Football1 week ago
Football1 week ago
 Crime4 days ago
Crime4 days ago
 Education6 days ago
Education6 days ago
 Crime1 week ago
Crime1 week ago