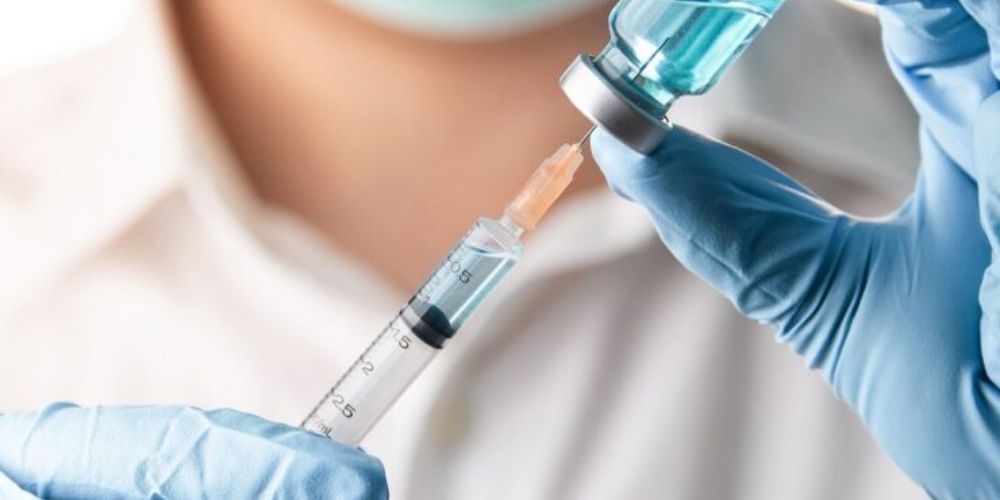
The federal government has issued a directive mandating that all federal tertiary hospital chief medical directors (CMDs) and medical directors (MDs) procure needles and syringes exclusively from NAFDAC-approved local manufacturers.
The directive, aimed at bolstering domestic production, was conveyed in a circular signed by the Minister of State for Health, Dr Tunji Alausa, on Friday.
Dr. Alausa said the directive was necessary for the survivial of local pharmaceutical industries that produce needles and syringes.
According to him, many of the domestic companies have faced severe challenges, with several having ceased operations due to the influx of substandard foreign goods into the market.
Consequently, the directive seeks to curtail the importation of foreign-manufactured needles and syringes and promote patronage of locally manufactured products.
In line with the directive, NAFDAC has been tasked with ceasing the issuance of licenses for importing foreign needles and syringes.
READ ALSO: NAFDAC uncovers fake cosmetics production syndicate in Lagos
Furthermore, companies involved in such importation activities will be delisted moving forward.
“All our tertiary hospitals are hereby directed to procure needles and syringes for your hospital needs from only the NAFDAC-approved local manufacturers,” Dr. Alausa said in the circular.
The approved local manufacturers include EL-Salmat Pharmaceuticals Company Ltd (Salmaject), HMA Medical Ltd (Deleject), and Afrimedical Manufacturing and Supplies Ltd.
Additionally, Dr Alausa provided a list of distributors for the approved manufacturers across various states in the Federation to facilitate easy procurement processes for the respective institutions.

 Entertainment6 days ago
Entertainment6 days ago
 Entertainment3 days ago
Entertainment3 days ago
 Comments and Issues6 days ago
Comments and Issues6 days ago
 Business7 days ago
Business7 days ago
 Health1 week ago
Health1 week ago
 Comments and Issues6 days ago
Comments and Issues6 days ago
 Health5 days ago
Health5 days ago
 Editorial Opinion1 week ago
Editorial Opinion1 week ago